
Visit Us
“It’s an amazing place to live; our alarm clock in the morning are the tigers roaring. At Australia Zoo, it’s not just looking at animals, you’re right in with them”
Robert Irwin
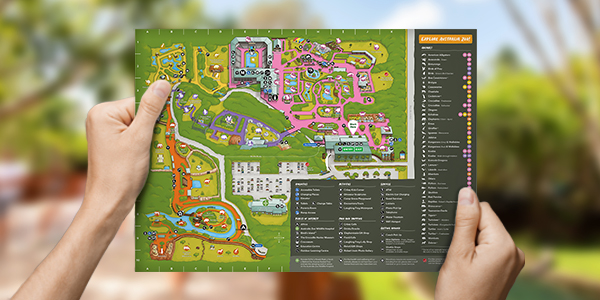
Zoo Map
Welcome to Australia Zoo’s Map. Please take your time to browse our amazing array of animals and attractions. This is a great starting point from which to map out your day!

Events Calendar
Every month is a celebration at Australia Zoo! Check out the AWESOME events coming soon. Each event is jam-packed with entertainment, rides, competitions, face-painting and stacks of family fun!

How to Get Here
Looking for helpful travel information? Whether you are driving, catching a bus or train or planning a luxury day tour directly from Brisbane, the Sunshine Coast or the Gold Coast. It’s all here!

Where to Eat
You won’t go hungry here! Our dining venues, located throughout Australia Zoo, cater for a wide variety of tastes and offer an extensive menu of fresh, hot and cold food and drinks.

Where to Shop
The Australia Zoo gift shops and online store offer a large range of gifts, Irwin family designer wear and Australian-made souvenirs. Don’t miss the new Stella the Pug range…..

Where to Stay
We have made it easy for you to organise your visit to Australia Zoo. Start planning your holiday to the Sunshine Coast and Australia Zoo today!

Plan your Day
We love teaching everyone about our amazing animals and have a stack of animal talks that will be sure to leave you with a greater understanding and appreciation for wildlife!

How to Prepare
To prepare yourselves for a full day of action-packed animal adventure, we recommend packing a few essentials for your day at the Zoo!

Services & Facilities
We want to ensure that your experience with us is a complete experience! Check out our extensive list of services and facilities to help plan your ideal Australia Zoo adventure.
